2012 International Conference on
Biomedical Ethics and East Asian Confucian Traditions
The Institute for Advanced Studies in Humanities and Social Sciences and the Center for Ethics, Law and Society in Biomedicine and Technology, National Taiwan University are very honored to welcome you to the 2012 International Conference on Biomedical Ethics and East Asian Confucian Traditions. This Conference will explore current bio‐medical ethical issues, in view of practice implementation and ethical frameworks, from the perspectives of Confucian approaches in East Asian context. Internationally and domestically renowned scholars in the field will give speeches either from a theoretical/analytical approach or from a practical/empirical approach to the conference theme, and to discuss implications for governance and regulation. Presentation topics include: biomedical ethics and Confucianism, Confucian bioethics methods, Confucian perspectives on informed consent, research ethics review, end of life care, assisted reproduction, medical education, risk governance and health communication, and medical law.
Date: March 23 (Friday) PM and 24(Saturday) AM/PM, 2012
Venue: Professor GB Chen Memorial Lecture Room, College of Pubic Health, National Taiwan University
Organizers:
- The Institute for Advanced Studies in Humanities and Social Sciences, National Taiwan University
- Center for Ethics, Law and Society in Biomedicine and Technology, National Taiwan University (CBME)
Co-organizers:
- College of Public Health, National Taiwan University
- College of Law, National Taiwan University
- Department of Social Medicine, National Taiwan University College of Medicine
- Charité Medical University, SIGENET Health Program, Berlin, Germany
- Taiwan Clinical Ethics Network (TCEN)
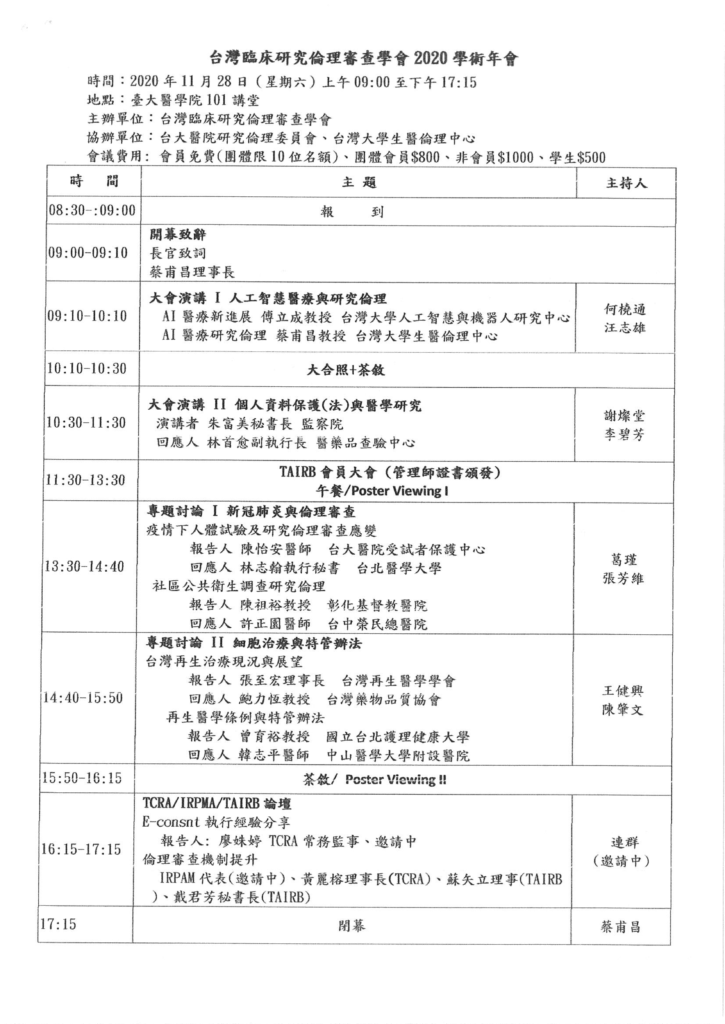
March 23
| Time |
Topic |
Speaker |
| 13:10-13:40 |
Registration |
| 13:40-14:10 |
Opening RemarkDeans Pan-Chyr Yang, Chun-Chieh HuangPhotography and Coffee |
| Session 1: Confucianism and Biomedical EthicsChair: Tzu-Yi Lin |
| 14:10-15:30 |
The Possible Implications of Confucianism to Biomedical Ethics |
Ynhui Park (South Korea) |
| The End of Life: Legal Change and Culture in Taiwan |
Tsung-Fu Chen (Taiwan) |
| Discussion |
| 15:30-15:50 |
Coffee break |
| Session 2: Confucian Approach to Biomedical EthicsChair: Jeu-Jenq Yuann |
| 15:50-17:40 |
The Fallacy and Dangers of Dichotomizing Cultures: Confucianism Misinterpreted in Chinese-Western Comparative Perspectives |
Jing-Bao Nie (New Zealand) |
| Principlism and Confucianism: Cultural Variances in Medical Ethics? |
Daniel Fu-Chang Tsai (Taiwan) |
| Salient Features of Confucian Ethics and Their Implications for Biomedical Issues |
Kam Por Yu (Hong Kong) |
| Discussion |
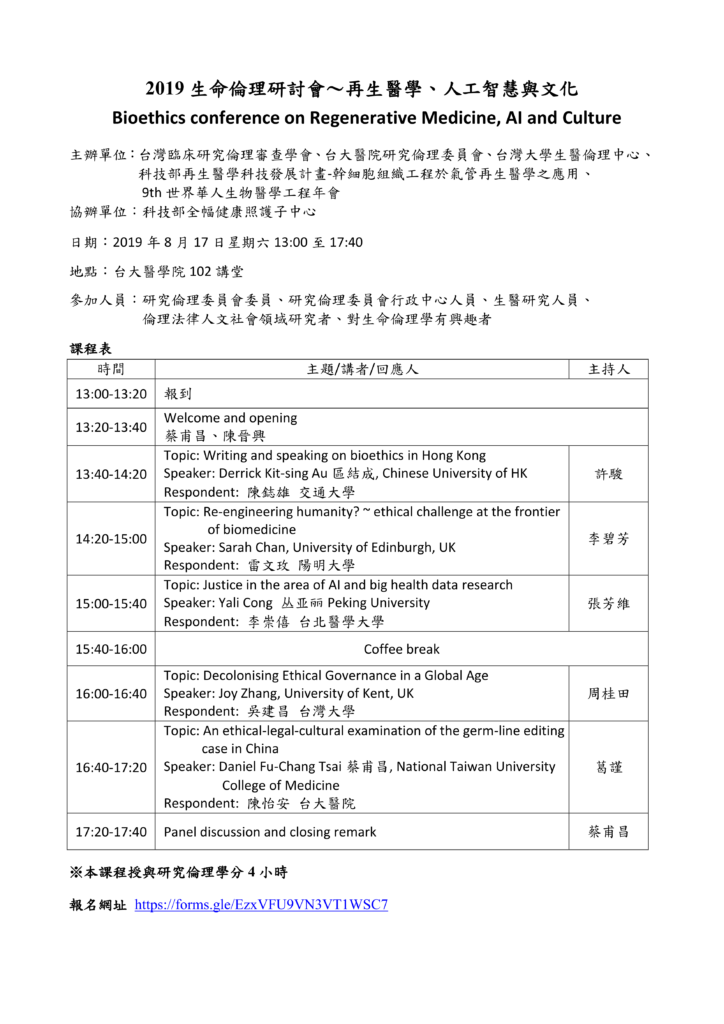
March 24
| Time |
Topic |
Speaker |
| 8:30-9:00 |
Registration |
| 9:00-9:10 |
WelcomeHong-Nerng Ho |
| Session 3: Research Ethics and ConfucianismChair: Hong-Nerng Ho |
| 9:10-10:30 |
Comparing and Complementing: Approaches to Bringing Confucianism to Life in Clinical Trial Ethics |
Ole Döring (Germany) |
| Ethical Review of Clinical Research |
Xiuqin Wang (China) |
| Discussion |
| 10:30-10:50 |
Coffee break |
| Session 4:Risk Communicationand Citizen DeliberationChair: Wei J. Chen |
| 10:50-12:10 |
The Deepening Distrustful Society without Risk Communication – A Comparison of Public Risk Perception on GMO in 2004 and 2011 in Taiwan |
Kuei-Tien Chou (Taiwan) |
| Citizen Deliberation on Surrogate Motherhood in Taiwan |
Kuo-Ming Lin (Taiwan) |
| Discussion |
| 12:10-13:10 |
Lunch |
| Session 5: Cross-Cultural Biomedical EthicsChair: Chi-Wan Lai |
| 13:10–14:30 |
Speaking Up in the East-Asian Ethical Tradition |
James Dwyer (Taiwan & USA) |
| A Cross-Cultural Study of Students’ Reasoning of Professional Dilemmas |
Ming-Jung Ho (Taiwan) |
| Discussion |
| 14:30-14:50 |
Coffee break |
| Session 6: Culture, Law and Biomedical PracticesChair:Jung-Der Wang |
| 14:50-16:10 |
What Role does Chinese Culture Play in Informed Consent |
Wei Zhu (China) |
| Law and Cultural Practices: The Implications of Taiwan Hospice and Palliative Care Act |
Kevin Chien-Chang Wu (Taiwan) |
| Discussion |
| 16:10-16:30 |
Closing remarks |
Ole Döring (Germany)Daniel Fu-Chang Tsai (Taiwan) |
| 16:30-17:30 |
Medical Humanity Museum Tour |